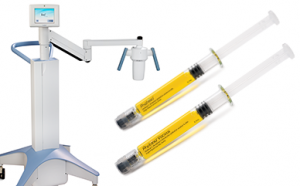
In April, 2016 Avedro of Waltham, Massachusetts received FDA Approval for its KXL® System for Corneal Cross-Linking and its riboflavin solutions (Photrexa® Viscous and Photrexa®) for the treatment of keratoconus and corneal ectasia.
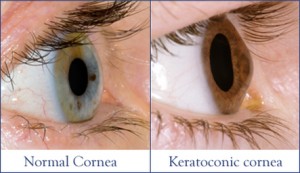
Keratoconus is a condition that affects the cornea, which is the transparent structure in the front of the eye. As keratoconus progresses, the normally spherical or globe-shaped cornea bulges in an irregular manner causing a more conical shape. A similar process happens in post-LASIK corneal ectasia. The abnormally bulging and conical shape of the cornea does not allow for proper focusing of light into the eye. People suffering with keratoconus and corneal ectasia have distorted, blurry vision with symptoms such as glare, haloes, or light sensitivity. Often specialized devices such as hard contact lenses (RGP lenses) are needed to help vision. Some patients with keratoconus or ectasia will progress to the point of needing a corneal transplant to restore their vision.
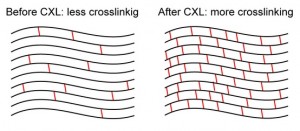 Collagen cross-linking (CXL) is an in-office surgical treatment that works by strengthening the corneal tissue to stop it from bulging. During the procedure, eye drops containing riboflavin (vitamin B2) are applied to the cornea and activated by ultraviolet light. This process strengthens (or crosslinks) the collagen fibers within the cornea. These crosslinks act as corneal anchors, preventing the cornea from further bulging into an irregular shape.
Collagen cross-linking (CXL) is an in-office surgical treatment that works by strengthening the corneal tissue to stop it from bulging. During the procedure, eye drops containing riboflavin (vitamin B2) are applied to the cornea and activated by ultraviolet light. This process strengthens (or crosslinks) the collagen fibers within the cornea. These crosslinks act as corneal anchors, preventing the cornea from further bulging into an irregular shape.
Originally developed in Germany, CXL has been used in Europe and around the world successfully for nearly 15 years. CXL was approved by the FDA in the United States in April 2016. Dr. Seth Meskin was part of a small group of doctors in the United States that served as an investigator for the FDA clinical trials on CXL. As such, Dr. Meskin performed several CXL treatments on patients as young as 13. He also has worked with colleagues from around the world and has years of experience caring for patients before and after their CXL procedures. Dr. Meskin was the first surgeon in the state of Connecticut to perform Corneal Collagen Crosslinking (CXL) with the FDA approved CXL device. This CXL device by Avedro is the ONLY CXL device currently approved for CXL in the United States.
For more information on CXL, keratoconus, and corneal ectasia, or to set up your appointment, please contact us at (203) 878-1236 in any one of our 4 offices in Milford, Orange, Branford, or Shelton. We are looking forward to hearing from you soon.